Overland Telegraph Wire
Reprinted from "INSULATORS - Crown Jewels of the Wire", June 1981, page 16
(These five pages are a reprint of the chapter "Overland Telegraph
Wire", from the book AMERICAN TELEGRAPHY. sorry we have lost track of who
sent it in -- we have had it a long time, and this is the first issue with space
enough to include it.)
CHAPTER XXXI.
OVERLAND TELEGRAPH WIRE.
IRON AND
HARD-DRAWN COPPER WIRE. -- MANUFACTURE OF, ETC. -- MECHANICAL TESTS AT FACTORY.
-- CONDUCTIVITY TEST. -- WIRE GAUGES, etc.
Until within a few years past,
iron was almost exclusively used for "overland" telegraph wires, although
it was well known that copper possessed electrical qualities far superior to
iron. But the former high price of copper, added to inherent mechanical defects,
combined, for years, to keep the latter metal out of the market, as a competitor
of iron for such purposes.
On this point the following language from an
advertisement which appeared in an electrical periodical in 1868, may be quoted:
"The superiority of copper as a conductor, over other metals, is well
known, and but for its ductility rendering its permanent suspension in a pure
state impracticable it would always have been used on telegraph lines."
The
tensile strength of "soft" copper is about one-tenth that of iron. The
ductility of soft copper is such that it becomes attenuated by its own weight
between poles; and having no elasticity, when elongated it has no tendency to
resume its previous form. As an electrical conductor, copper is seven times
better than iron. Again, self-induction is much less marked in copper than in
iron; thus, apart from its superior conductivity, copper is better adapted for
rapid signaling than iron.
There is no comparison as between copper and iron in
the matter of durability under exposure to, and without artificial protection
from, the elements. Indeed, copper may be said to be, under ordinary atmospheric
conditions, practically incorrodible; whereas it is known that iron even when
protected by galvanizing, will succumb to the attacks of moisture and acids
within ten or twelve years; in some places in less than one year, as, for
instance, in the vicinity of factories and railroad yards. Copper wire, exposed
to the same conditions, simply takes on a coating of oxide and soot and is not
further attacked.
About 25 years ago the price of copper was at least 10 times
greater than iron. More recently, however, the discovery and development of
large deposits of comparatively pure copper in this country conduced to a very
material reduction of the cost of that metal, and a marked improvement in the
manufacture of copper wire also soon followed.
This improvement consisted in
providing a wire known as "hard-drawn" copper wire, of much greater
purity, and one possessing a much higher tensile strength, that formerly;
although the added strength was obtained at the cost of pliability, which
however, is not found to be seriously, if at all, detrimental.
Prior to
this improvement in the manufacture of copper wire, an effort had been made to
provide a telegraph wire which should have the strength of iron and much of the
conductivity of copper. This resulted in the production of a
"compound" wire of iron or steel, and copper, many miles of which were
strung in this country. In some instances the copper was placed over the iron
wire by electrolytic deposition; in others, by placing the copper, in strips,
spirally around the steel core, the edges being run together so that the seams
were not perceptible.
Siliconized copper wire and phosphor-bronze wire were also
introduced for the same purpose, but neither these, nor the compound wire, has,
in this country, given anything like the satisfactory results obtained by the
use of hard-drawn copper wire, which is doubtless explainable by the fact that
the tensile strength given to hard-drawn copper wire, in the process of
"drawing," having been found ample for the purpose of overland lines,
it was evident that it would possess, practically, all the mechanical advantages
of the compound wire and siliconized wire, with, in addition, superior
electrical qualities. Its cost also, is probably below either of the foregoing
mentioned wires.
In some instances it was found that the durability of the
compound wire was impaired by a separation of the two metals.
In silicious
bronze wire, which is an alloy of copper and tin, the silicon is mainly used to
aid in the removal of impurities, especially oxides and sub-oxides, and is not
intended as a part of the alloy. The tensile strength of silicious bronze wire
is somewhat greater than that of hard-drawn copper, but the former appears to
lose in conductance as it gains in tensile strength. Silicious bronze wire has
been somewhat extensively used in Europe, but in this country it has not been
employed, other than experimentally, for telegraph purposes.
In the year 1884
the extensive employment of hard-drawn copper wire for overland telegraph
purposes was begun in this country.
Although some misgivings were felt that the
experiment, (for such its employment at first was conceded to be), would meet
with failure, yet the advantages to be derived from its use, if it should prove
successful, were so numerous, and decided, that the experiment was tried, and
with such abundant success that today, in this country, it may be said that
copper wire for overland telegraph lines is rapidly superseding iron. Indeed,
iron wire is now, by some companies, only employed on new lines as a means of
strengthening the lines.
This successful use of copper had apparently shown that
the high percentage of elongation always formerly called for in the
specifications for telegraph wire was unnecessary; the percentage of
elongation of hard-drawn copper wire not exceeding, on an average, 2.5 per cent,
and in may cases falling below .5 of 1 per cent, without any marked prejudicial
results following.
THE MANUFACTURE OF IRON AND COPPER WIRE.
The iron
mostly used in the manufacture of wire is Swedish iron. It is brought to this
country in the shape of pig iron, which, after passing through various processes
to remove impurities is rolled into rods of any desired size. It is then
prepared for the process of "drawing," by which it is made into wire.
This preparation consists of first thoroughly cleansing the rods by washing, or
"pickling," them in acids, after which they are covered with a flour
paste, which is then dried hard by baking in an oven. The process of
"drawing" consists of pulling the rods, while cold, by powerful
machinery, through a steel die, in the manner indicated in Fig. 388.
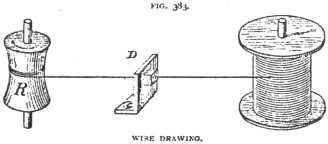
In the
figure, D is the die, R is a revolving drum, around which the wire is wound as
it comes attenuated through the die. The rod is started through the die by
filing the end for a short distance, when a clamp is attached to it. This clamp
is fastened to a chain, and the latter to the Drum R. R is revolved by machinery
not shown in the figure. The drawing is repeated until the wire is reduced to
the desired size, a smaller die being used at each drawing. During the drawing
process the wire becomes hardened and, consequently, it is necessary to anneal
it between each drawing, and as the drawing wears off the flour coating, the
wire must be re-coated between each drawing. It is said to be a curious fact
that the wire in passing through the die does not come into contact with it at
all, the flour acting also as a lubricant.
The dies are made of the
hardest obtainable steel or specially prepared cast iron.
When iron wire has
been drawn to the size required it is then annealed to the desired degree of
softness. Each coil of the wire is then carefully inspected by the workmen to detect
flaws or defects of any kind; coils containing which are rejected.
The next
process to which the iron is subjected is that of galvanizing. This consists of
covering the wire with a thin coating of zinc. The object of this is to protect
the iron from rusting, that is, from oxidizing. This the zinc does by combining
with the oxygen of the air and thus forming a covering of oxide of zinc over the
wire, which is not further assailed by air, unless in the presence of a gas,
such as sulphuric acid gas, set free from burning coal, etc., when the acid
combines with the oxide of zinc forming sulphate of zinc. The latter, being
soluble in water, is soon washed off the wire, leaving the iron to be quickly
attacked by the oxygen of the air, and in a short time corroded.
It is very
essential that the surface of the wire should be chemically free from all
impurities, such as sand, scales, cinder, oxides, etc, before it is galvanized,
otherwise the zinc will not properly adhere to the iron. To insure this
essential, the wire is again "pickled" by immersion in a vat
containing a solution of dilute sulphuric acid, for from six to twenty-four
hours, after which it is flushed in water to remove the acid. To still further
cleanse the iron it is immersed in muriatic acid which removes oxides that form
(after the pickling process) when the wire is exposed to the air.
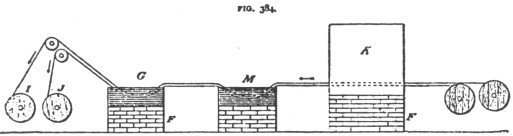
Galvanizing Iron Wire.
The act
of galvanizing the iron wire consists in momentarily immersing the wire in a
bath of molten zinc. One of the methods employed for this purpose is shown in
Fig. 384. The wire is brought on reels to the vicinity of a sort of oven K,
which has, running through it, horizontally, a number of fire brick tubes, which
are kept at a white heat by a furnace F, extending under the oven. M is a trough
containing a solution of muriatic acid. G is a bath of molten zinc. The Zinc is
kept "boiling" by a furnace under the trough. Several reels of wire
may be run simultaneously through the tubes of K. The wire is passing through
these tubes at a moderate rate of speed becomes heated to redness. On its exit
from its tube the wire falls into the acid, where all traces of grease, oxides,
etc., are removed, and the next moment the wire passes through the molten zinc
and emerges there from galvanized.
The wires are automatically would
on the reels I, J, after leaving the zinc bath.
The iron being heated to a high
temperature in passing through the tubes any acid that may adhere in passing
through the solution is at once evaporated and the distance between the acid vat
M and the zinc bath G is so short that but little time is given for the iron to
oxidize. It is very important that the zinc should be kept at a fixed
temperature; the best results are said to be obtained with a bath heated to
about 740 degrees F.
The effectiveness of the galvanizing is tested, generally,
as follows: A piece of the galvanized wire is immersed for one minute in a
saturated solution of sulphate of copper. The affinity of the sulphuric acid of
the salt for zinc is well known. The effect of this immersion is that some of
the zinc combines with the sulphuric acid of the sulphate setting free copper.
When iron is immersed in such a solution the copper is set free on the iron.
The foregoing action is repeated three or four times, as may be called for in
specifications. If at the end of the fourth immersion there is no appearance of
a copper deposit on the wire thus repeatedly immersed, but, on the contrary, it
remains black, as after the first immersion, the galvanizing may be considered
effective. The presence of a copper deposit would indicate that the iron had
become exposed and that, consequently, the galvanizing was imperfect.
Copper rods are prepared for drawing into wire in the same general way as iron.
The manner of drawing the copper rods into wire and that wire into still finer
wire is also similar to that by which iron is "drawn." When, however,
the copper wire is intended to have a high tensile strength it is not annealed
so frequently between the different drawings as in the case of iron.
Experiments
have shown that the ductility of copper wire decreases as it tensile strength
increases, but the experiments were not continued to an extent sufficient to
show the exact ratio. A specimen of copper wire, thoroughly annealed, .128 inch
in diameter, was found to have tensile strength of 330 lbs., and elongated 36
per cent. A sample of the same wire, on being drawn twice, to reduce its
diameter to .104 inch, had a tensile strength of 330 lbs., and elongated 23 per
cent. Another specimen, from the same piece, on being drawn thrice to bring it
to the same diameter, namely .104 inch, was found to have a tensile strength of
415 lbs. and elongated but 3 per cent. Still another specimen from the same
wire, drawn four times to reduce it to .104 inch, had a tensile strength of over
550 lbs. and elongated but 1 per cent. The average of a number of like
experiments indicated that, in obtaining an elongation of 2.5 per cent. to 3
per cent., a reduction of 130 to 140 lbs. in the tensile strength would follow.
The term "hard drawn" is applied to distinguish the unannealed from
the annealed copper wire; the only difference between soft copper wire and hard
drawn copper wire being that one is annealed after drawing while the other is
not. The process of drawing the wire through the die forms a thin, hard,
polished crust, or shell, not exceeding the one thousandth of an inch in
thickness, over the wire. Inside of this crust the metal is, seemingly,
comparatively soft. The tensile strength of hard drawn copper wire appears to
rest in this outside shell, for the lightest indentation made around the
circumference of the shell with a sharp instrument will at once lower its
breaking strain; and while, with an undented surface, the copper wire may
withstand 5 or 6 bends on itself, with such a dent it will break in one bend.
WIRE JOINTING AT FACTORY. -- At one time it was quite customary to require, in
specifications for telegraph wire, that the wire should be delivered in
continuous lengths of one half mile or more, without joints. This was when it
was the habit to make the large twist joint (shown Fig. 420). In ordering hard
drawn copper, also, the same requirement was inserted; the "sleeve"
joint being then used. The objections to such joints were that the tensile
strength at those points was considerably less than that of the main wire; that
they retarded the work of uncoiling the wire in the act of stringing, and that,
when the wires were strung, the joints frequently, by engaging with parallel
wires, caused steady crosses, which would otherwise have been but momentary wind
crosses. Hence, it was very desirable to reduce the total number of such joints,
to a minimum.
As is is not an uncommon occurrence for wire to break in the act
of drawing, the matter of jointing such broken wires in such a manner as to
avoid the objections referred to, was one which received much attention from the
manufacturers, and various attempts were made to weld the joint, mechanically,
without materially increasing its bulk, or decreasing its tensile strength; but
only with indifferent success. Of late, however, electric welding has been
resorted to, for this purpose, with marked satisfactory results. In making
joints, or welds, by this process, the ends of the broken wires are brought
together, and are fastened to separate clamps. Wires connected with a dynamo
machine are brought to these clamps, and a very strong current is then caused to
pass through the tips of the broken wire, which speedily produces a heat
sufficient to form a perfect union between them. For ordinary telegraph wire the
time of application of the current is but a fraction of a second, but the time
of application of the current, the extent of the wire exposed between the
clamps, and the pressure with which the ends are brought together, varies with
different wires. Welds made in this way have scarcely a perceptible burr, and
tests have shown that the tensile strength of the weld is practically similar to
that of the wire proper.
|
